The Epigenome
Featuring Dr. Dana Dolinoy, UofM School of Public Health professor and faculty director of the Epigenomics Core at Michigan Medicine.
Happy February! This semester, I am taking Environmental Health Sciences (EHS) 660, a graduate-level course on environmental epigenetics and its role in public health. Taught by the amazing Dr. Dana Dolinoy, this class examines the principles and applications of epigenetics and how they relate to nutrition, environmental exposures, and disease etiology.
I was fortunate enough to ask Dr. Dolinoy a few questions about epigenetics in order to further our understanding of this revolutionary topic.
Check it out below!
Q: How do you explain epigenetics to someone unfamiliar with this concept?
Dana Dolinoy: Health researchers and scientists who are investigating human diseases typically evaluate the intersection of three factors: genes or DNA, environment including toxicants, diets, and stress, and how these change over time or age. Oftentimes researchers will use twin studies to hold genetics constant in order to evaluate environmental effects. On the flip side using analytical approaches we can try to hold the environment constant to better evaluate genes. What’s become clear over the last several years is that there is another factor at play – even after carefully controlling for genetics, environmental exposures, and lifestyle changes, monozygotic twins with the same DNA still display widely varying presentations of diseases, including cancer, and also inbred animal strains. Thus, researchers have begun to investigate the epigenome.
Epi means “over” or “on top of,” so you can interpret this as the instruction book that tells genes when to turn on, where to turn on, and how much to turn on.
The more formal definition is heritable changes in gene expression due to the DNA being marked or modified but not due to changes in the underlying DNA sequence
Analogies: Our genome is like a computer’s hard drive, it contains a lot of data, but that data just sits there until the epigenomic software comes along and tells us what to do. In fact, every cell in our body has the same DNA - the hardware in this analogy - but it is the epigenetic software that helps determine a hair cell, liver cell, or heart cell.
Another analogy is the punctuation in a sentence. The order of the words in a sentence may be the same, but the addition of a comma alters its meaning, such as Let’s Eat, Dad or Let’s eat Dad.
Q: Sometimes it can be difficult for some individuals to understand real-life examples of epigenetic changes. Can you provide a few common examples of epigenetic manifestations in humans? For example, lead and BPA are environmental toxicants, but how do they impact our bodies, and what are the common conditions associated with their exposure?
DD: The emerging field of toxicoepigenetics evaluates how chemical exposures such as metals like Pb or endocrine-disrupting chemicals such as bisphenol A or BPA interact with our epigenome to affect gene expression.
It turns out that exposures, especially in development, can change an epigenetic mark called DNA methylation (DNAm), which is simply a quartet of atoms, one carbon and three hydrogens that sit down on DNA and control the level at which our genes are expressed. It’s sort of like a dimmer on a switch on a light. A gene that lacks methylation is greatly expressed but when that gene gains methylation, it is more dimly expressed.
One important issue about the epigenome from a public health perspective is that, unlike the genome which is static and not modifiable, the epigenome is dynamic and potentially modifiable. So we may be able to use things like nutrition or healthy lifestyles to counteract the negative effects of chemicals on our epigenome.
But it’s not just toxicants that can affect the epigenome - In the mid-2000s, important animal-based work conducted at McGill University in Montreal identified that maternal care in the window just after birth can alter DNAm patterns in the offspring's brain. Pups that were born to mothers with low levels of maternal care grew up to have anxiety and be poor mothers themselves. And the exact opposite for pups born from mothers with high levels of maternal care. What the researchers did next was also interesting – if the pups of lowly attentive moms were infused with pharmaceuticals that erased DNAm on the glucocorticoid gene then the anxiety was reduced. So the effects of early maternal care are reversible, at least in an animal model.
Other studies of humans have shown that traumatic events such as war or abuse can have effects on DNAm not only in the exposed generation but also in subsequent generations. For example, both mothers and babies of mothers who developed PTSD in response to the September 11 World Trade Center attacks had altered cortisol levels compared with mothers who did not develop PTSD and their babies, which the authors proposed could be due to altered epigenetic effects. Other studies link child abuse with altered epigenetic profiles in stress response genes.
These types of findings in humans are still in the early stages and need to be repeated. One of the issues in working with human populations is that we need to use so-called biologically available DNA from sources like blood or saliva and these might not be representative of the target tissue of interest like the brain.
Returning to the WTC attacks, animal model researchers have shown that mice exposed to dust collected from the WTC showed epigenetic changes in the lung. Whether these changes are also present in the blood would greatly help inform human studies.
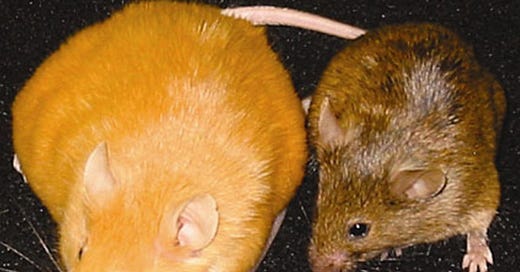
Q: Can you share more about your work with the Agouti mice (pictured below) and its implications in humans?
DD: To help me convey why the epigenome is so important for our health and disease, I’m going to turn to two of my favorite furry friends - the Agouti Mice.
These two mice don't look much alike, do they? One's brown, one's yellow . . . one's trim, the other looks like it could stand to knock off a few ounces. Well, it might surprise you to learn that these are in fact sisters, and not just sisters, but identical twins.
In the yellow, overweight mouse, the Agouti gene went unmethylated – it was turned on all the time when it should've been off. That caused her to feel less satisfied after eating, so she just kept pigging out. But with the brown mouse, that gene is completely methylated and shut down, so she feels full and stays slender.
You've probably heard of the chemical Bisphenol-A, or BPA. It's all over the place: in food and beverage containers, baby bottles, and dental sealants. As a result, it's also detectable in about 95% of Americans.
In one study, we used the Agouti mice to explore whether a mother's exposure to BPA could alter the fetal epigenome, and eventually the long-term fate of her offspring.
When we fed the pregnant mothers BPA, we noticed that the number of yellow, obese offspring went way up. We concluded that the BPA was turning the Agouti gene on when it should have been off.
Then we ran a second study, where pregnant mice were still exposed to BPA, but this time they were also given a nutritional supplement -- a common ingredient found in soy products. Sort of a "more-Asian" diet." The results? More brown, thin mice. This suggests that the soy supplement was counteracting the negative effects of the BPA.
The Agouti mice have been used to evaluate other exposures…. Lead, radiation, alcohol.
Q: How can epigenetics be used to conquer diseases like cancer or Alzheimer’s? Is there any concrete progress toward utilizing epigenetics to cure or reverse certain conditions?
DD: Before people began to understand the importance of the epigenome, health and disease were thought to be mostly affected by heredity and the environment. For example, when identical twins develop differently, it must be a result of either different environments or different behaviors, such as diet or smoking, or exercise. But our mice showed us that a third factor besides nature and nurture – the epigenome – plays a huge role, too. And what's so powerful about the epigenome is that although it's stable, it can be improved – for example, to make us less vulnerable to harmful things in the environment . . . like BPA.
The context of the environmentally induced epigenetic change is very important. In fact, alterations can be adaptive or neutral in addition to negative.
This is one of the issues with current epigenetic-based therapies, such as DNAm inhibitors used in certain end-stage cancers. They demethylate not only the tumor suppressor genes we want to be expressed but also all regions.
Q: Are certain groups more susceptible to epigenetic changes (race, ethnicity, gender, socioeconomic status (SES), age)?
DD: This is a great question - there have been differences identified by race and SES, but less so connecting to a specific endpoint.
Read more here.
Q: Can epigenetic changes be passed down to future generations?
DD: Maybe! There is a fair amount of evidence that the preconception and perinatal periods are particularly vulnerable for the next generation. What is less clear is if there are lasting effects on the next generations and beyond. When a pregnant (mammal) female is exposed during pregnancy, three generations are simultaneously exposed - the mother, the child, and the child’s germline, which will become the grandchild. There is some toxicological evidence that exposure can transmit to the 4th generation, which is unexposed, but this field is still emerging and hard to study across generations in humans!
Check out this interesting website.
Q: What changes or policies can be implemented to limit drastic changes to one’s epigenome?
DD: Traditional risk assessment is not an easy fit for epigenetics because sometimes an environmentally induced change is beneficial or inert and sometimes a negative epigenetic change is transitory.
An interesting example – in humans – of the potential for offsetting benefits from positive environmental exposures and the reversibility of epigenetic changes comes from the year-long NASA Twins study, which evaluated physical, molecular, and cognitive changes from exposure to space. An epigenomics arm of this study evaluated the epigenome of twins, Mark and Scott Kelly. It showed how the space environment altered DNA methylation and resulted in epigenetic changes in the Space Twin (Scott) in flight, which reverted to baseline upon return to Earth. The majority of Scott’s DNA methylation changes were observed from the second six months of space exposure, indicating that timing and duration, not just the fact of exposure, must be considered.
Q: What motivates you to study and research epigenetics?
DD: Epigenetics is a hopeful science and one that lends itself to public health prevention. Unlike the genome which is static and unmodifiable, the epigenome is dynamic and potentially modifiable. If we can identify biomarkers of exposure we may someday be able to intervene - with nutrition or pharmacological approaches to reverse a risky endpoint.
Billions of dollars went into the Human Genome Project. But without the corresponding Human Epigenome Project – and it's only getting a fraction of what the Genome Project received – we really only have the "hard drive half" of the picture. We're not studying and improving the "epigenomic software" that can put all that genomic knowledge to work.
Thank you so much to Dr. Dolinoy for taking the time out of her very busy schedule to answer these questions and share her insight with us on epigenetics in public health.
Dr. Dana Dolinoy serves as the NSF International Chair of Environmental Health Sciences and is a Nutritional Sciences and Environmental Health Sciences professor at the University of Michigan School of Public Health. She is the faculty director of the Epigenomics Core at Michigan Medicine and leads the Environmental Epigenetics and Nutrition Laboratory. Dr. Dolinoy received her Ph.D. in Genetics and Genomics and Integrated Toxicology from Duke University and her MSc in Public Health from Harvard University.
Questions or comments? Email me at reemlfawaz@gmail.com or fawazr@umich.edu.